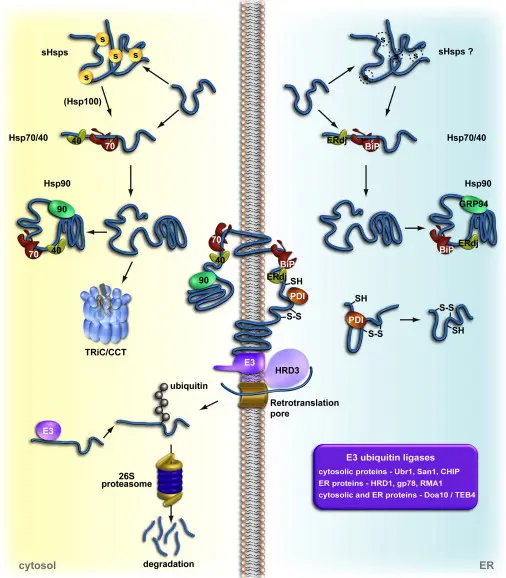
The Proteasome – A Key Player in Cellular Protein Degradation The proteasome is a large protein complex in eukaryotic cells that is tasked with degrading and recycling unwanted, damaged, or misfolded proteins. This helps to balance proteins and regulate such vital cellular processes as the cell cycle, gene expression, and development.
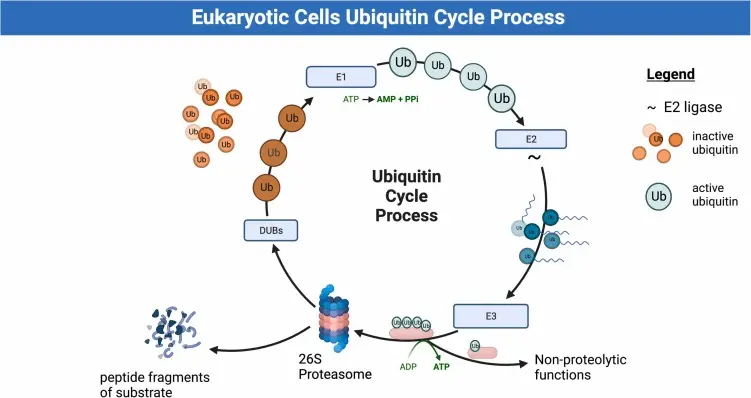
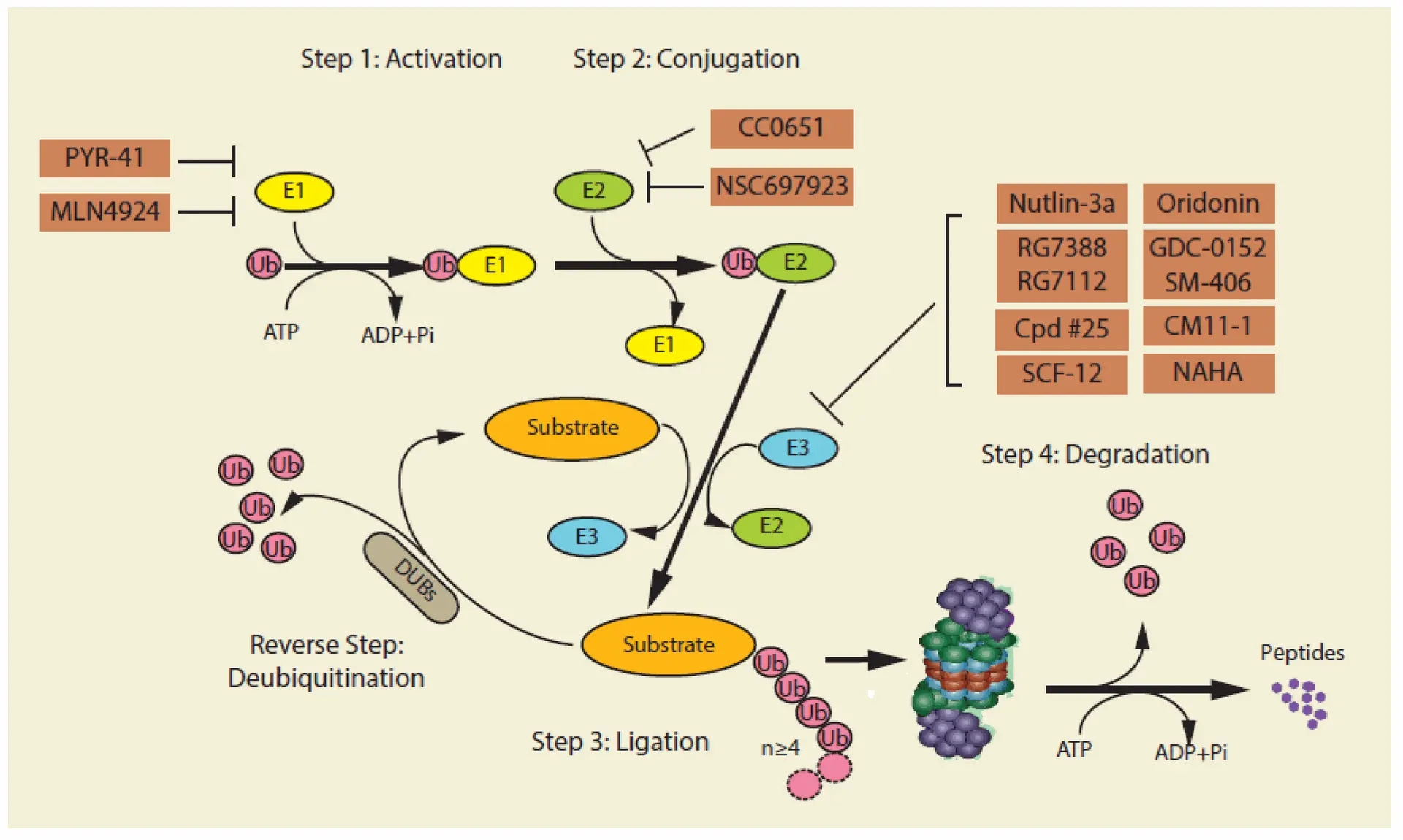
Proteins bound for breakdown are first tagged with a small protein ubiquitin, causing the creation of a polyubiquitin chain. The tag directs them to the 26S proteasome, a multi-subunit enzyme with a 20S core and regulatory particles. Active sites of protein breakdown are within the 20S core, while the regulatory units recognise and denature the tagged proteins in an ATP-dependent fashion.
By selective protein breakdown, the proteasome plays a central role in cellular control and quality maintenance. Its dynamic function is particularly critical in processes like stress response, immune signaling, and developmental transitions.
Why protein degradation is critical for cellular homeostasis.
Protein degradation is vital to cellular homeostasis because it ensures the elimination of irreparably damaged, misfolded, or excess proteins that can otherwise accumulate and lead to dysregulation of normal cell function. This controlled process of degradation prevents toxic protein aggregation, maintains the balance of the level of key signalling molecules, and allows the cell to rapidly adapt to environmental shifts. Through the specific degradation of regulatory proteins, such as the proteins that govern the cell cycle, transcription, or stress, the cell is able to fine-tune its internal processes. In addition, protein degradation also recycles amino acids for resource conservation and to allow efficient biosynthesis. On the whole, this mechanism is accountable for protein quality and homeostasis a fundamental aspect of cellular health and survival.
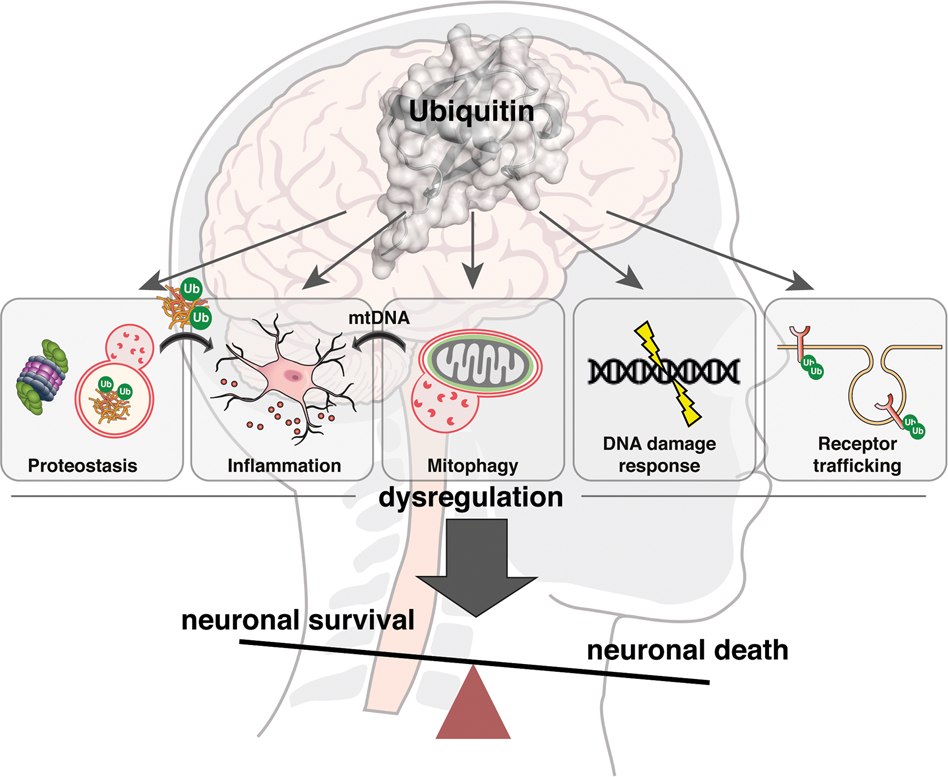
Dysfunction of the ubiquitin-proteasome system (UPS) is implicated in a range of diseases, most notably certain cancers and neurodegenerative disorders, where the balance of protein degradation is critically disturbed.
The Ubiquitin Proteasome System in Neurodegenerative Diseases
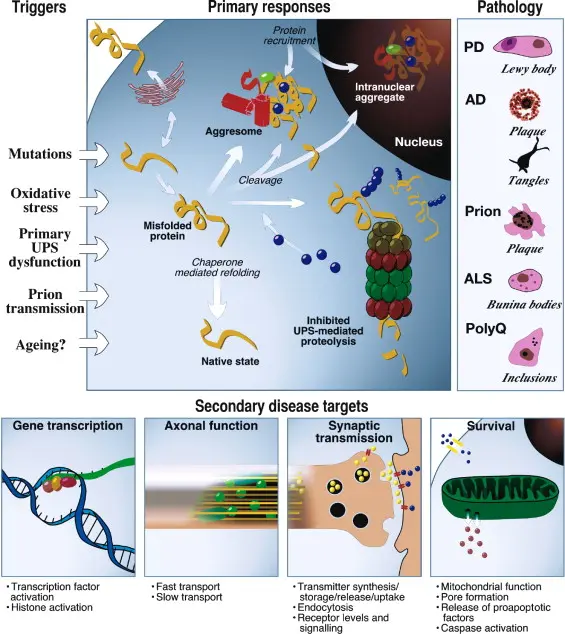
Neurodegenerative diseases like Parkinson’s and Alzheimer’s are mainly marked by a malfunctioning ubiquitin-proteasome system (UPS), which results in the inadequate breakdown of harmful proteins. In Parkinson’s disease, the inability to properly degrade misfolded α-synuclein causes it to build up into Lewy bodies—structures that are toxic to neurons and contribute to their degeneration. Similarly, Alzheimer’s disease is characterized by the accumulation of amyloid-beta plaques and tau protein tangles, partly caused by disrupted proteasomal degradation. These protein clumps impair normal neuron function, leading to cognitive decline and memory impairment.
How Altered Ubiquitin-Proteasome Activity Drives Tumor Development
In cancer cells, dysregulation of the ubiquitin-proteasome system (UPS) plays a critical role in promoting tumor progression by affecting processes such as cell cycle control, DNA repair, apoptosis, angiogenesis, and gene transcription. This occurs through the abnormal degradation of tumor suppressor proteins like p53 and p27, as well as the stabilization of oncogenic factors such as NF-κB. Central to this system are ubiquitin-activating enzymes (E1), including UBA1 and UBA6, which are essential for managing cellular stress responses like DNA damage. Altered expression of these enzymes has been linked to cancers such as lung carcinoma and cutaneous squamous cell carcinoma. Additionally, the human genome encodes numerous E2 conjugating enzymes, many of which are overexpressed in cancers and correlate with poor patient prognosis. For example, UBE2L3 is upregulated in non-small cell lung cancer, where it facilitates the ubiquitination and degradation of p27 via interaction with the E3 ligase Skp2. The UPS also comprises over 700 E3 ligases and deubiquitinases (DUBs), which regulate protein stability in cancer. Notable E3 ligases like MDM2 negatively regulate p53, while DUBs such as USP7 modulate p53 function by either stabilizing p53 directly or enhancing MDM2 activity. Overexpression or mutation of these enzymes contributes to tumor growth and resistance to therapy, making them important targets for cancer treatment.
Ubiquitin-Proteasome System Inhibitors: Strategies and Challenges in Cancer Therapy
The clinical success of proteasome inhibitors (PIs) like Bortezomib has positioned the ubiquitin-proteasome system (UPS) as a vital target in cancer therapy, especially in malignancies such as multiple myeloma and mantle cell lymphoma that heavily rely on UPS-mediated protein degradation. Currently, three PIs—Bortezomib, Carfilzomib, and Ixazomib—are approved for clinical use, with newer second-generation drugs like Oprozomib and Marizomib undergoing trials to improve pharmacokinetics and reduce neurotoxicity. However, limitations such as resistance development and systemic toxicity remain significant challenges.
Beyond the proteasome, inhibition of E1 ubiquitin-activating enzymes, such as UBA1 and UBA6, offers broader therapeutic effects by disrupting key cellular pathways, including DNA repair and NF-κB signaling. Promising agents like MLN4924 (Pevonedistat) and TAK-243 have entered clinical trials for leukemia and solid tumors, although toxicity and trial discontinuations have impacted progress.
Targeting E3 ligases—enzymes responsible for substrate specificity in ubiquitination—is also gaining traction. MDM2, a negative regulator of p53, is a prime example, with several small-molecule inhibitors in clinical testing. However, the structural complexity and lack of defined active sites make E3 ligases difficult to drug. As a result, technologies like PROTACs (proteolysis-targeting chimeras) have emerged, enabling selective protein degradation by hijacking E3 ligases to tag disease-associated proteins for destruction.
Additionally, modulating deubiquitinases (DUBs), which reverse ubiquitination, is a promising avenue. DUB inhibitors aim either to suppress oncogenic DUBs or enhance tumor suppressive activity. Although some, like VLX1570, faced setbacks due to toxicity, other candidates such as Mitoxantrone and 6-thioguanine are being tested in clinical trials for cancers including leukemia.
Modulating Protein Modification Pathways in Neurodegenerative Disease Therapy
SUMOylation, a process that modifies proteins, plays an important role in neurodegenerative diseases (NDs), but research results have been inconsistent, and suitable animal models are still limited. Despite this, scientists are exploring SUMOylation as a potential therapeutic target. For example, ginkgolic acid, a natural compound that blocks SUMO activation, can reduce harmful huntingtin protein levels and boost autophagy in Huntington’s disease (HD) cell models. Another compound, 2-D08, inhibits the SUMO-conjugating enzyme UBC9 and protects cells from amyloid β toxicity, which is linked to Alzheimer’s disease. It may do so by binding tightly to toxic forms of amyloid β, preventing damage.
On the other hand, some studies suggest that enhancing SUMOylation can also be beneficial. Certain small molecules that activate SUMOylation have shown neuroprotective effects in cell studies. In stroke research, a temporary increase in SUMOylation near damaged brain tissue appears to help protect and repair cells. Experiments with mouse neural stem cells also show that boosting SUMOylation helps them turn into neurons more effectively after brain injury.
While these findings are promising, no drugs that specifically target SUMOylation are currently in clinical trials for neurodegenerative diseases. Further studies are needed to better understand its therapeutic potential.